I am pleased to publish a guest post from friend and colleague, Dr. William Dillon, an interventional cardiologist in Louisville Kentucky. (He can be followed on Twitter @wmdillon.) I offer his words in the hope that they will bolster awareness of heart disease and foster a spirit of cooperation among all Kentuckians.
Bill and I trained together at Indiana University. After IU, we have been partners in private practice for more than a decade. He is the plumber; I the electrician.
In addition to his keen sense of clinical judgement and finely tuned hand-eye skills in the cath lab, Dr. Dillon has also felt the urge to do more to impact heart disease.
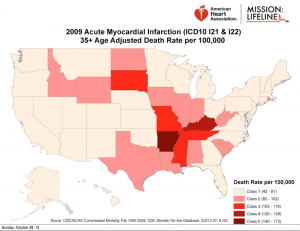
He has long been an innovator and facilitator of urgent PCI for acute myocardial infarction–heart attack. His guest post addresses the need to improve our state’s dismal ranking in heart attack care. Only Arkansas does worse than Kentucky.
High quality care of acute MI is so about teamwork. Done well, urgent PCI brings out the best in US healthcare. It’s like an orchestra–everyone plays an important role. But for too many citizens of this wealthy nation, a discrepancy in care exists. Bill wants this to change. Good for him.
Here is an excerpt from his post….
…Not every hospital has the ability to perform this life saving procedure. PCI centers require a dedicated ER, transport, nursing cath lab personnel and an interventional cardiologist on call 24-hours a day. Speed is critical. Every 1-hour delay in opening the blocked artery increases the death rate by 10%. The longer a coronary artery remains closed the more the heart is damaged.
Unfortunately, outside of major metropolitan areas access to PCI is limited. Too many patients do not receive any reperfusion treatment, or, they receive a treatment that is inferior to a timely PCI. This needs to change.
It is time to set up a regional network to transfer AMI patients to dedicated PCI centers throughout the state. This type of network exists for trauma patients and has been tried in North Carolina for heart attacks with great success.
To read the entire post, head over to In the Prime: An urgent call to improve heart attack care in Kentucky.
JMM
One reply on “In the Prime post up — An urgent call to improve heart attack care in Kentucky”
Today’s News on Reversal Agent for Pradaxa
Pradaxa®- Development of antidote may expand range of methods to reverse anticoagulant effect during emergency situations
06th November 2012 11:01 IST
Ingelheim, Germany (Business Wire (Business Wire India))
New findings presented at the 2012 American Heart Association Scientific Sessions in Los Angeles provide early indications that a highly specific and selective antidote currently in development can, when approved, deliver safe and effective rapid reversal of the anticoagulation effect of Pradaxa® (dabigatran etexilate). If needed, the potential antidote may be used during critical care situations or where existing reversal strategies may not be sufficient.*1 Following the successful completion of the early research phase, the progression into phase 1 clinical trial development was initiated for the investigational antidote (a fully humanized monoclonal antibody fragment – Fab).
The investigational antidote should be regarded as an additional option for patient management in critical care situations and may support the established reversal strategies already available in emergency medicine. In research, the Fab holds promise as one of the first specific antidotes developed for any of the novel oral anticoagulants (NOACs) used for stroke prevention in atrial fibrillation (AF).1
A risk of bleeding is a known possible treatment complication of all anticoagulant therapies used for stroke prevention in AF.2 Even in the absence of a specific antidote, both doses of dabigatran etexilate demonstrated significantly lower life-threatening and intracranial bleeding than warfarin during the landmark RE-LY® clinical trial programme. Additionally, significantly lower major and fatal bleeding events were experienced with dabigatran etexilate 110mg bid.3,4
Further to existing reversal strategies, Boehringer Ingelheim is developing a specific antidote to dabigatran. Driven by the company’s commitment to scientific innovation, the antidote is expected to provide physicians with an additional option for management of bleeding during critical care situations where rapid reversal is required.
The results from the preclinical studies show the Fab to offer:1
Very tight binding affinity to dabigatran molecules – high specificity with no effect on other molecules including warfarin
Rapid, dose-dependent decrease in experimentally-induced blood loss, sustained for up to 6 hours after intravenous injection
Safe reversal of the anticoagulation effect of dabigatran (demonstrated in ex vivo clotting tests)
The management of severe bleeds in clinical practice remains the same for all anticoagulant treatments,5,6 with dabigatran having the additional option of removal from the blood system via hemodialysis.7
No specific fast-acting antidote is currently available to reverse the anticoagulant effect of any of the NOACs or warfarin.6 In the case of warfarin, vitamin K is frequently misunderstood as an antidote while it is actually a supplement, which simply replaces the vitamin K needed for the synthesis of the coagulation factors blocked by warfarin. Reversal of the anticoagulant effect of warfarin is a rather slow and complex procedure which may take up to 36 hours.8
Boehringer Ingelheim remains dedicated to advancing science and ensuring that physicians have all the tools they may require to effectively manage critical care situations, improving the overall benefit of treatments and optimising patient management. As such, the development of the Fab progressed to phase I clinical trials.
“These new findings are encouraging and demonstrate the potential of an antibody for rapid reversal of the anticoagulant effect of Pradaxa®, restoring a patient’s coagulation. Although emergency situations are relatively rare in clinical practice, Boehringer Ingelheim is committed to ensuring the best brain protection for people living with atrial fibrillation, which includes ensuring that physicians have every option they may require in the critical care situation,†stated Dr. Joanne van Ryn, Department of CardioMetabolic Disease Research, Boehringer Ingelheim.
It is widely accepted that the benefits of anticoagulant treatment for stroke prevention in AF far outweigh the risk of bleeding.9,10 The benefits of Pradaxa® have been recognised worldwide, leading to widespread regulatory approvals and recent reconfirmation of the positive benefit/risk profile by the European Medicines Agency.11,12 Clinical trial and real-life experience of dabigatran now equates to over one million patient-years in all licensed indications and exceeds that of all other novel oral anticoagulants used for stroke prevention in AF.11,13
* Reversal of Anticoagulant Activity of Dabigatran and Dabigatran-induced Bleeding in Rats by a Specific Antidote (Antibody Fragment) Lead Author: J. van Ryn, Oral Presentation 9928
http://www.boehringer-ingelheim.com/news/news_releases/press_releases/2012/05_november_2012dabigatranetexilate